Our previous technical tip spoke about silylation reagents that may be used to derivatize a range of polar, acidic, and basic analytes to improve volatility and peak shape during gas chromatography. Silylating reagents are not the only game in town when derivatizing amino groups and carboxylic acids for GC analysis. Primary halides and chloroformates are two reagents that are common when derivatizing analytes for HPLC, and they may be easily adapted to sample preparation workflows for GC.
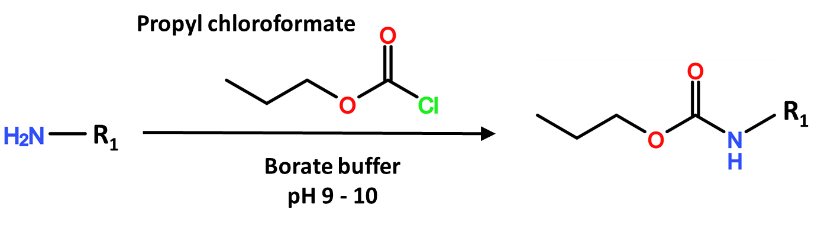
 Chloroformate reagents target primary and secondary amines to generate carbamates, for which a common chloroformate reagent for GC is Propyl chloroformate. Prepare the chloroformate reagent in an aprotic water-miscible solvent, such as acetonitrile, and separately prepare a buffer of sodium borate with pH 9.0 -10.0. Sodium borate is the preferred buffer to minimize side reactions of the chloroformate reagent, but other basic pH buffers may be used. The reaction generally calls for equal volumes of buffer and chloroformate reagent solution, both of which are small enough to fill half of a scintillation vial when combined. Shake the vial for about 1 – 5 minutes at room temperature to complete the reaction. The trick is the clean-up, for which hexane may be directly applied to the aqueous reaction mixture to perform a liquid-liquid extraction. Hexane will separate as the top layer that will contain the derivatized analytes, and which is easily accessible to a pipette.
Chloroformate reagents target primary and secondary amines to generate carbamates, for which a common chloroformate reagent for GC is Propyl chloroformate. Prepare the chloroformate reagent in an aprotic water-miscible solvent, such as acetonitrile, and separately prepare a buffer of sodium borate with pH 9.0 -10.0. Sodium borate is the preferred buffer to minimize side reactions of the chloroformate reagent, but other basic pH buffers may be used. The reaction generally calls for equal volumes of buffer and chloroformate reagent solution, both of which are small enough to fill half of a scintillation vial when combined. Shake the vial for about 1 – 5 minutes at room temperature to complete the reaction. The trick is the clean-up, for which hexane may be directly applied to the aqueous reaction mixture to perform a liquid-liquid extraction. Hexane will separate as the top layer that will contain the derivatized analytes, and which is easily accessible to a pipette.
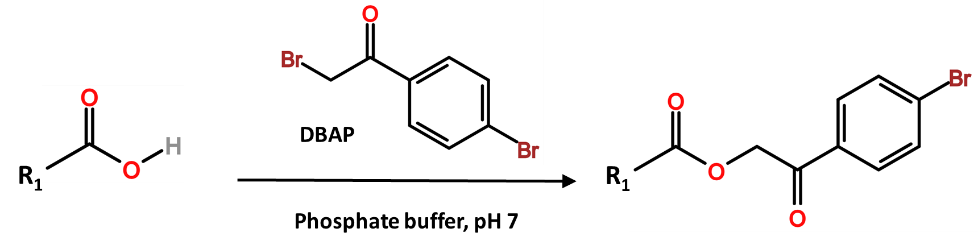
 Primary halides may be used to derivatize carboxylic acids into esters. Two common reagents are 2,4’-Dibromoacetophenone (DBAP) and Pentafluorobenzo bromide (PFB-Br). The advantage of PFB-Br is the selectivity and sensitivity of the derivatized analytes towards ECD detectors, while DBAP also allows for ECD detection. The reaction conditions are comparable to chloroformate conditions above, including a liquid-liquid extraction to capture the derivatized analyte. The primary halide reagent is dissolved in an aprotic solvent that is water-miscible. The targeted carboxylate analyte may be treated with a neutral phosphate buffer, to which the derivatizing reagent solution is then added. If the reagent solvent is water miscible, then the reaction may proceed with or without an additional alkylamino catalyst, and the resulting ester may then be extracted into hexane. One consideration is that the derivatization may take at least an hour and may require elevated temperatures.
Primary halides may be used to derivatize carboxylic acids into esters. Two common reagents are 2,4’-Dibromoacetophenone (DBAP) and Pentafluorobenzo bromide (PFB-Br). The advantage of PFB-Br is the selectivity and sensitivity of the derivatized analytes towards ECD detectors, while DBAP also allows for ECD detection. The reaction conditions are comparable to chloroformate conditions above, including a liquid-liquid extraction to capture the derivatized analyte. The primary halide reagent is dissolved in an aprotic solvent that is water-miscible. The targeted carboxylate analyte may be treated with a neutral phosphate buffer, to which the derivatizing reagent solution is then added. If the reagent solvent is water miscible, then the reaction may proceed with or without an additional alkylamino catalyst, and the resulting ester may then be extracted into hexane. One consideration is that the derivatization may take at least an hour and may require elevated temperatures.
There are many varieties to the general protocols shared above, particularly when working with PFB-Br. The derivatizing reagent may intentionally be dissolved in a water-immiscible solvent, depending on the broader considerations of the sample itself. The important consideration is that you choose the protocol that aligns with the broader chemical characteristics of your analyte and the sample matrix with which your experiment begins.
