GC Technical Tip
Level: Basic
Good column selection: Polarity vs Selectivity
When it comes to selecting the best GC column for your analysis, understanding the concepts of polarity and selectivity is crucial. While often used interchangeably, they have distinct meanings in the context of gas chromatography. Let's explore how you can make informed decisions for column selection.
Overview
Polarity and selectivity are often regarded as interchangeable, however in practice they are quite different when looking at gas chromatography. Selectivity refers to the difference in retention factors between two peaks whilst polarity is an assigned value specific to a GC column, based on an assignment using solute-solvent properties. With a plethora of GC phases available to an analyst, how can you ensure you are selecting the best stationary phase for your method?
Tip
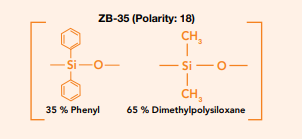
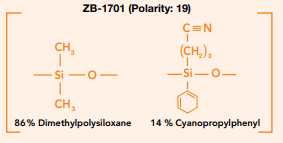
Resolution between compounds is primarily determined by the selectivity of the stationary phase, and your choice will have the largest effect on this. Column selectivity is unique to column polarity, but it is important to also ensure the polarity of the phase you select is appropriate to the compound types you are analyzing. For example, if you have a selection of mid-polarity compounds generally these will resolve best on a mid-polarity column. What is important when selecting a stationary phase is to consider that two columns with almost equivalent polarity can offer very different selectivities. If we consider the ZebronTM ZB-35 and ZB-1701, which have polarity indexes of 18 and 19 respectively, due to their very different stationary phases they work in different ways depending on the class of compound in question.
Using PAHs as an example we can see the different selectivity profiles for these hugely aromatic compounds, with the ZB-1701 and its higher level of aromaticity giving better resolution, specifically of the critical pair of dibenzeno[a,h]acridine and dibenzeno[a,j]acridine (Fig. 1) when run using the same column dimensions and temperature profile.
Fig. 1 ZB-35
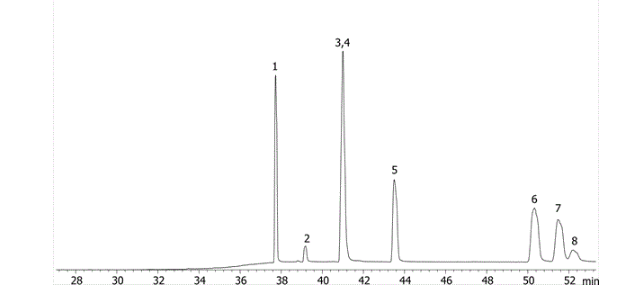
Fig. 2 ZB-1701
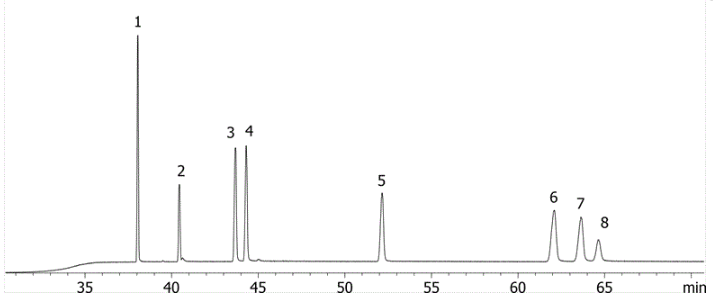
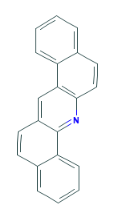
Critical Pair (3,4)
3 - dibenzeno[a,h]acridine
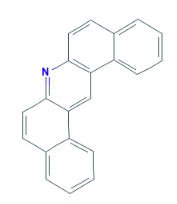
4 - dibenzeno[a,j]acridine
In GC, 3 types of interactions are important for selectivity:
- Van der Waals Interactions, which occur between non- polar groups on the phase and non- polar compounds.
- Dipole-dipole interactions, which can assist with separating compounds with similar boiling points where their chemical structures are different.
- Hydrogen bonding, which can be a powerful tool for polar compounds. It can also lead to poor peak shape if not controlled appropriately.
When selecting the most appropriate GC column for your application, polarity should be considered first, and using a column which matches your compound polarity is generally advised. Once this is selected, elution order of your compounds is primarily determined by boiling point and in general compounds with lower boiling points will elute earlier than those with higher boiling points. The caveat to this is when the lower boiling compound has more interaction with the stationary phase than the higher boiling compound. For a purely boiling point based separation you would be looking to use a stationary phase such as the ZB-1 which is 100% dimethylpolysiloxane – where Van De Waals forces form the main interaction between the analytes and the stationary phase.

On Demand Webinar
Exploring Fast GC Solutions for Priority Pollutants like PAH and PCB in Food and Environmental Samples.
Trademark
Zebron is a trademark of Phenomenex.


