HPLC/UHPLC Technical Tip
Level: Intermediate
How can capacity factors drive method robustness?
When looking at data generated from HPLC analysis, it is natural to focus on retention time, peak asymmetry and efficiency. These parameters will allow the user to identify and quantify analytes: the more precise this information is the greater the confidence in peak identity and analyte concentration.
Capacity factor is often an overlooked parameter of separations, but when utilized correctly can guide the user to produce more robust methodology. The capacity factor serves to normalize the retention time of an analyte to the dimensions of the analytical column, and is defined as:
K’ = tr - t0/t0
Where tr is the retention time of the analyte, and t0 the elution time of an unretained compound.
The importance of capacity factor can best be illustrated by an example:
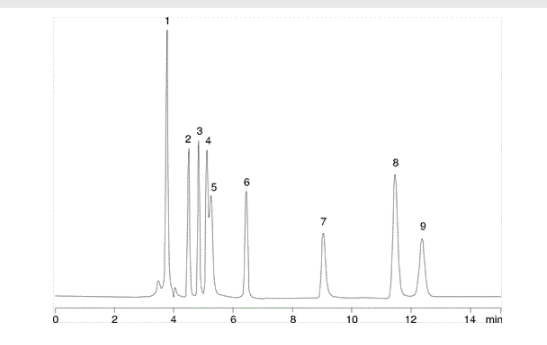
1. Oxalic acid
2. Tartaric acid
3. Glycolic acid
4. Formic acid
5. Pyruvic acid
6. Malonic acid
7. Acetic acid
8. Maleic acid
9. Citric acid
Here we have a separation of organic acids, run on a C18 column which is stable to 100% aqueous conditions. The mobile phase is 20 mM potassium phosphate buffer at pH 2.9, with a flow rate of 0.7 ml/min. At first glance oxalic acid is retained at almost 4 minutes, but upon looking at this a little more closely the t0 for this column is approximately 3.7 minutes. When this is considered it is clear that oxalic acid, a very polar diacid, is barely retained by the column at all.
K’ = 3.9 - 3.7/3.7 = 0.054
This indicates that oxalic acid spends almost no time associating with the stationary phase during its transit through the column. In such cases analyte retention will be greatly influenced by small, unintentional changes in parameters including:
• Mobile phase composition
• Mobile phase pH
• Sample diluent strength
• Temperature
Such a method is therefore unlikely to be robust, with oxalic acid retention varying from run to run, column to column and from one batch of mobile phase to another.
This leads to the recommendation, whenever methods are being developed, that capacity factors should lie in the range of 2 - 10. We have seen the potential for problems with capacity factors of less than 2, but why stop at a capacity factor of 10?
During an isocratic run, peak width increases with increasing retention time due to diffusion. Whilst the use of smaller particle columns and optimal flow rates will help to reduce this diffusion, it cannot be eliminated. Additionally, high capacity factors ultimately result in long run times, which are generally undesirable in a productive laboratory environment.
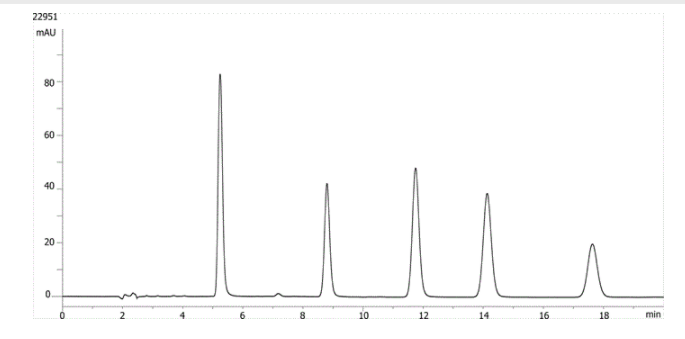
1. Hydrocortisone
2. Corticosterone
3. 11-a-Hydroxyprogesterone
4. Cortisone acetate
5. 11-Ketoprogesterone
Here we have an example of steroids run on a 150 x 4.6 mm column at 1 ml/min. 11-etoprogesterone elutes at around 17.5 minutes, with a capacity factor of approximately 10.6. Peak broadening is evident in this isocratic run, indicating that the latest eluting peak is being retained on column for an excessive period of time.
Understanding the guideline (capacity factors of between 2 and 10) is helpful, but leaves us with the question, how can they be adjusted?
The capacity factor is inversely related to the elutropic strength of the mobile-phase. A higher elutropic strength will lower the capacity factor, and vice versa. When low capacity factors cannot be increased by reducing the elutropic strength of the mobile phase then it may be necessary to switch to a stationary phase that can offer greater retention of the analytes, for example by using a more polar stationary phase for polar analytes.


