HPLC/UHPLC Technical Tip
Level: Basic
The effects of gradient on buffer capacity
HPLC gradients are a fantastic means of analyzing a sample for which the analytes exhibit a wide range of retentive strengths along the stationary-phase of your preferred column. Gradients are not always accommodating towards buffers when attempting to maintain a specific pH. If the buffer is limited to your aqueous mobile-phase while the organic mobile-phase remains strictly an organic solvent, then the gradient is prone to solubility limitations and inconsistent buffering strength as the ratio of organic solvent increases during the gradient. This may be overcome by applying an even distribution of the buffer towards both the “weak” and “strong” mobile-phases of your method, for which we will review some approaches and examples.
How about spiking both the “weak” and “strong” mobile-phases with an acidic or basic additive, such as the formic acid (HCO2H) at 0.1% (v/v)?
The spike in both mobile-phases will ideally maintain a consistent level of the additive in the overall mobile-phase as the ratio of “weak” and “strong” mobile-phases alters during the gradient. The shortcomings of such a spike are that the additive may be prone to volatilization from the mobile-phase mixture, while also inducing inconsistent strengths of protonation or deprotonation towards analytes. Perhaps some of us can admit to having been less than precise in our preparation of (v/v) spikes at one time or another. Alternatively, a proper buffer with the corresponding salt for a specific additive will precisely maintain the consistent pH at which it is prepared without the risk of the additive volatilizing.
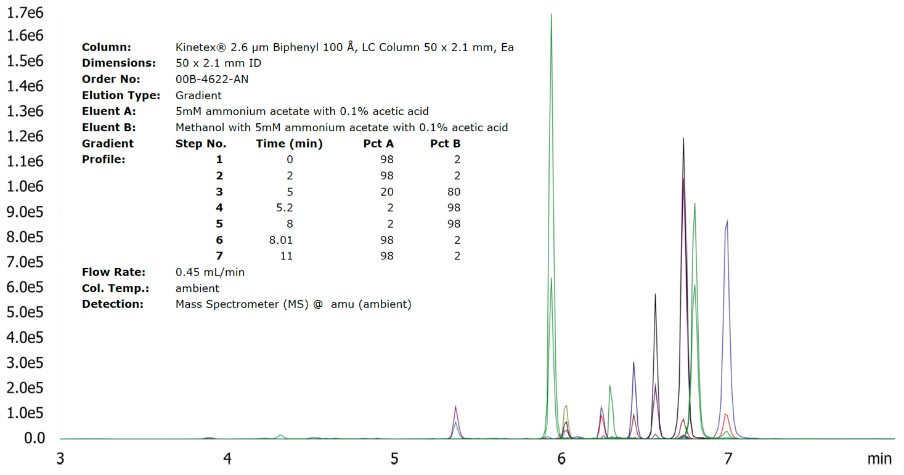
Ammonium formate (NH4HCO2) and ammonium acetate (NH4CH3CO2) buffers are the most amenable towards gradients, as these salts are soluble in methanol, while the corresponding aqueous buffers may be mixed with high ratios of acetonitrile. The necessary buffering strength typically ranges only 5 – 10 mM in each mobile-phase, but compare this with an isocratic method of 60:40 “20 mM buffer”/acetonitrile, for which the buffer concentration relative to the overall mobile-phase is only 12 mM. Figure 1 features an analysis of Mycotoxins on a Kinetex™ Biphenyl with 5 mM ammonium acetate buffers comprising both the aqueous and methanol mobile-phases, and for which the ammonium acetate was dissolved directly in the methanol.
Acetonitrile will need to be mixed with an aqueous buffer of common buffering reagents to maintain the solubility of the buffering salt. Figure 2 features the HILIC-mode analysis of underivatized amino acids on a Biozen™ Glycan, for which a stock buffer of 100 mM ammonium formate buffer (pH 3.1) was spiked into either water or acetonitrile in a 9:1 ratio of solvent/buffer. The resulting mobile phases each featured overall buffer concentrations of 10 mM ammonium formate, allowing the gradient to maintain a consistent overall buffering strength.
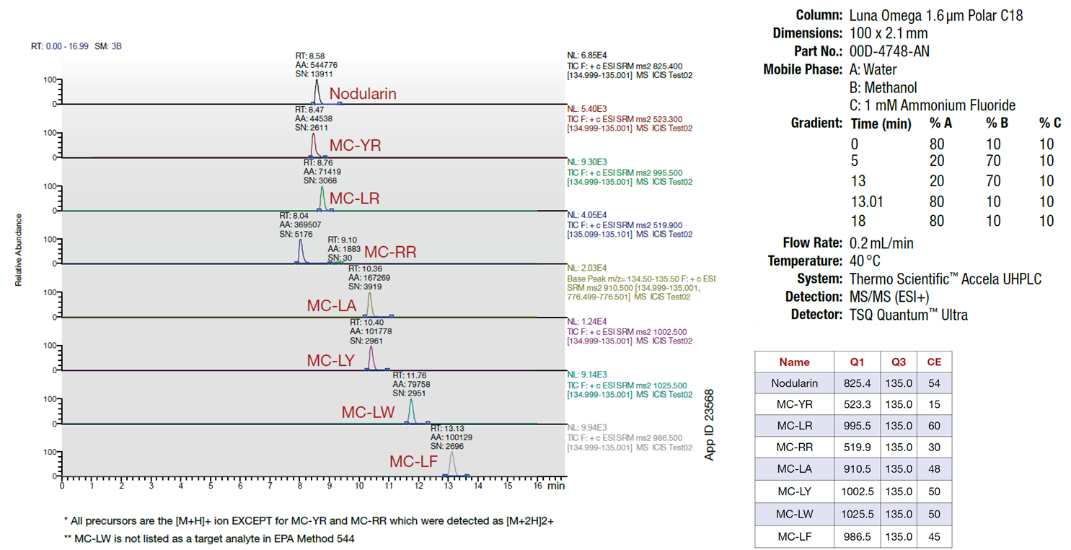
Ammonium fluoride (NH4F) is often included in small amounts (0.1 – 0.2 mM) in the overall mobile-phase to generate highly sensitive fluoride adducts [M+F]- during ESI-negative mode LC/MS, or to encourage the generation of either [M+H]+ or [M+NH4]+ ions in ESI-positive mode. Figure 3 demonstrates the use of a third solvent line by which a 1 mM NH4F buffer is constantly delivered at a ratio of 10% of the overall mobile-phase, corresponding to 0.1 mM in the overall mobile-phase throughout the gradient. Ternary and quaternary pumps facilitate this method, but an initial isocratic hold is recommended at the beginning of the method to accommodate the transferability of the method among instruments. Ternary and quaternary gradients are subject to dwell volumes that are specific to each instrument, and can be a concern even when one of the solvent lines is maintained at a constant percentage of the overall gradient.
Be mindful of solubility considerations when attempting to incorporate either ammonium bicarbonate buffers or phosphate buffers into a gradient, particularly when working with phosphate buffers. These buffers run the risk of precipitation when blended with high percentages of organic solvents, and a solubility study is recommended. Lastly, be sure to include your intended mobile-phase buffer as a portion of your sample diluent to align the pH of your mobile phase, which will benefit the peak shape and reproducibility. Using a gradient where the mobile phase has a consistent buffering strength and pH value will ultimately improve the reproducibility of the LC method.
Trademarks
Kinetex, Luna, and Biozen are trademarks of Phenomenex.