Gas chromatography is a fantastic technique with which to achieve exceedingly sharp peaks and resolution within a complex mixture of analytes, but each analyte must be volatile to facilitate GC analysis. Derivatization provides a means to analyze analytes that are otherwise difficult to volatilize. Such analytes include acids and bases, but also entail high-boiling neutral analytes that have a large number of polar substituents, such as sterols and small sugars. Silylation is a common and feasible means of derivatizing highly polar analytes to facilitate GC analysis.
Silylating reagents are simple means by which to derivatize a wide range of analytes, most commonly sugars, phenols, weak acids, amines, amides, and thiols. 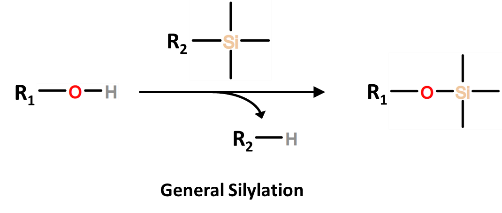 Generally, a silylating reagent will replace an “active” hydrogen of a polar functional group with a non-polar TMS (trimethylsilane) group. The resulting TMS derivative of the targeted analyte will have a lower polarity and a lower boiling point (specifically a higher vapor pressure). The resulting derivative will volatilize more easily and will be amenable to a wide range of GC stationary-phases.
Generally, a silylating reagent will replace an “active” hydrogen of a polar functional group with a non-polar TMS (trimethylsilane) group. The resulting TMS derivative of the targeted analyte will have a lower polarity and a lower boiling point (specifically a higher vapor pressure). The resulting derivative will volatilize more easily and will be amenable to a wide range of GC stationary-phases.
TMSI (N-trimethylsilylimidazole) is a common reagent for targeting hydroxyl groups and carboxylic acids, for which the imidazole leaving group is suited for scavenging the active hydrogen. TMSI can also target phenols and thiols. The silylating reagents BSA and BSTFA feature stronger leaving groups, allowing these reagents to derivatize amines, amides, and amino acids in addition to the analytes targeted by TMSI. Common silylating reagents are suitable for addressing “active” hydrogens that are not sterically hindered. TMCS (also abbreviated as TMS-Cl) is a smaller silylating reagent that may be added as a catalyst for other silylating reagents to improve the silylation of sterically hindered groups. TMSC is generally not used on its own during the derivatization of GC samples.
Catalysts and polar aprotic solvents are often used to enhance the susceptibility of the targeted “active” hydrogen to replacement with a TMS group, such as the use of pyridine to enhance the leaving capacity of the active hydrogen from a phenol. 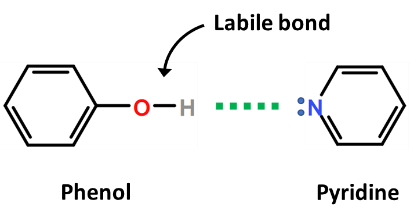 Silylation reactions are often performed either “neat” or in an aprotic solvent (organic solvents that do not have any hydroxyl groups). Protic solvents (commonly alcohols or water) will react with the silylating reagent.
Silylation reactions are often performed either “neat” or in an aprotic solvent (organic solvents that do not have any hydroxyl groups). Protic solvents (commonly alcohols or water) will react with the silylating reagent.
Avoid GC stationary-phases that include hydroxyl groups, such as “WAX” phases, as these will react with any unused silylating reagent, and can also potentially react with the silylated analytes themselves. Low-polarity and moderately-polar siloxane-based phases are recommended for the analysis of silylated analytes. As for caring for your GC instrument, be mindful of the volatility of the byproduct that is generated by the leaving group of the silylating reagent when preparing a GC sample.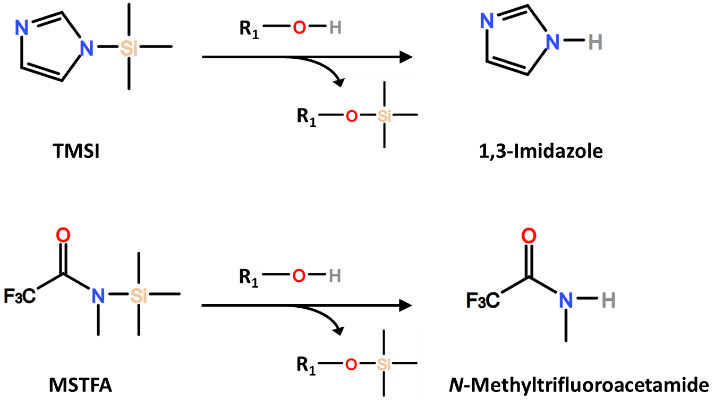 Imidazole (byproduct from TMSI) is an active base and has an atmospheric boiling point of 256 °C, while N-methyltrifluoracetamide (byproduct from MSTFA) is a neutral amide that has a boiling point of 156 °C. Regardless of the silylating reagent, change the inlet liner and trim the first few centimeters from the GC column once peak shapes begin to exhibit tailing and loss of sensitivity.
Imidazole (byproduct from TMSI) is an active base and has an atmospheric boiling point of 256 °C, while N-methyltrifluoracetamide (byproduct from MSTFA) is a neutral amide that has a boiling point of 156 °C. Regardless of the silylating reagent, change the inlet liner and trim the first few centimeters from the GC column once peak shapes begin to exhibit tailing and loss of sensitivity.
There are many silylating reagents from which to choose, some of which are more specific to certain functional groups than others. Be mindful of the silylation catalysts and reaction conditions that are particularly favorable to certain analytes, and be aware of the retention times of the silylating reagents themselves during analysis. Please feel welcome to share any questions, and I thank you for your consideration.
